



Theme
Clinical Engineering
INSTITUTION
Imam Abdulrahman Bin Faisal University
The goal of this project is to design and build an alternative device to the invasive glucometer that is reliable, fast, painless, and cost-efficient to smoothen the experience of patients. This non-invasive device uses multi-wavelength Near-Infrared (NIR) spectroscopy to detect glucose concentration levels in the blood.
Diabetes is a metabolic disorder disease marked by deficiencies in insulin secretion or inappropriate body cells’ response to insulin, which leads to elevated blood glucose levels outside of the normal range [1].
The criticalness of diabetes is strikingly growing on a global level. According to a study made in 2017 by the International Diabetes Federation, there are 425 million adult diabetic patients worldwide, and around 3,852,000 of diabetic cases were found in Saudi Arabia [2], as shown in Figure 1.
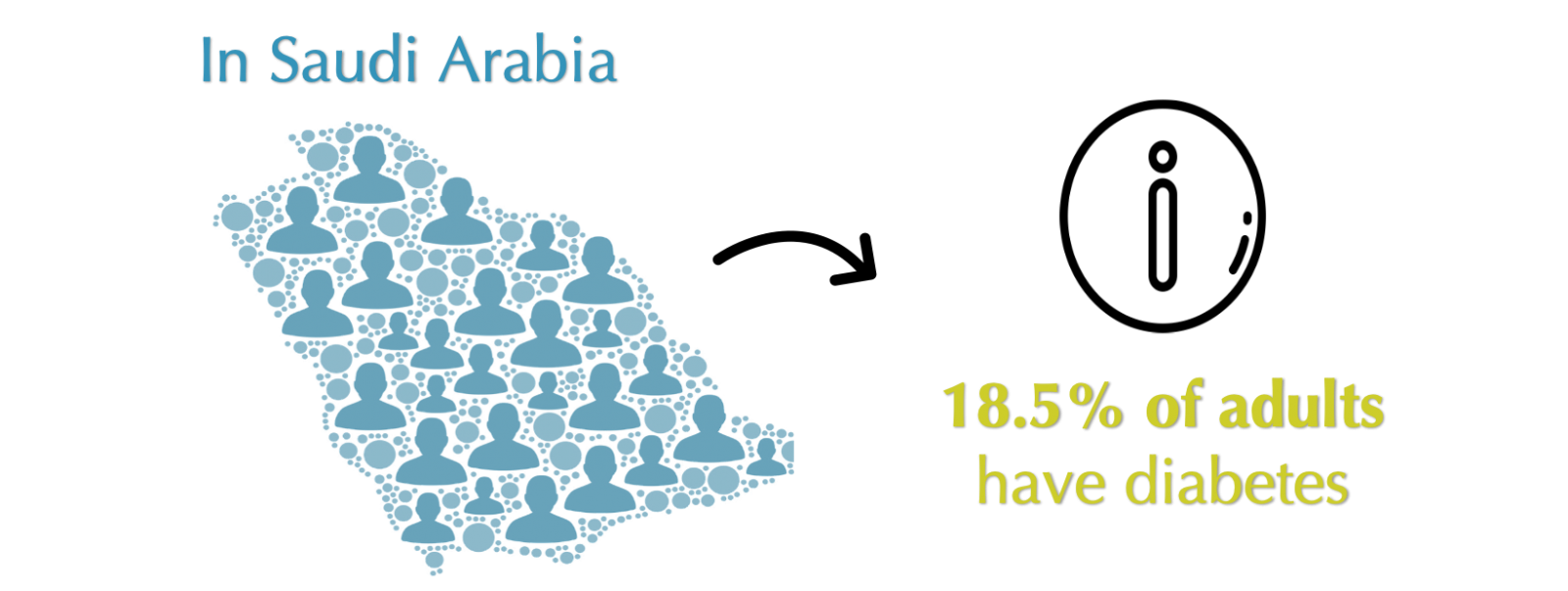
Figure 1: Prevalence of diabetes in adults in Saudi Arabia.
Frequent glucose monitoring is necessary to prevent diabetes complications. The traditional method currently in use requires punctuating the skin in order to acquire a drop of blood to be tested. Although it is considered to be accurate, it has multiple drawbacks for being invasive, painful, promoting infection, and using cost-intensive blood glucose testing supplies.
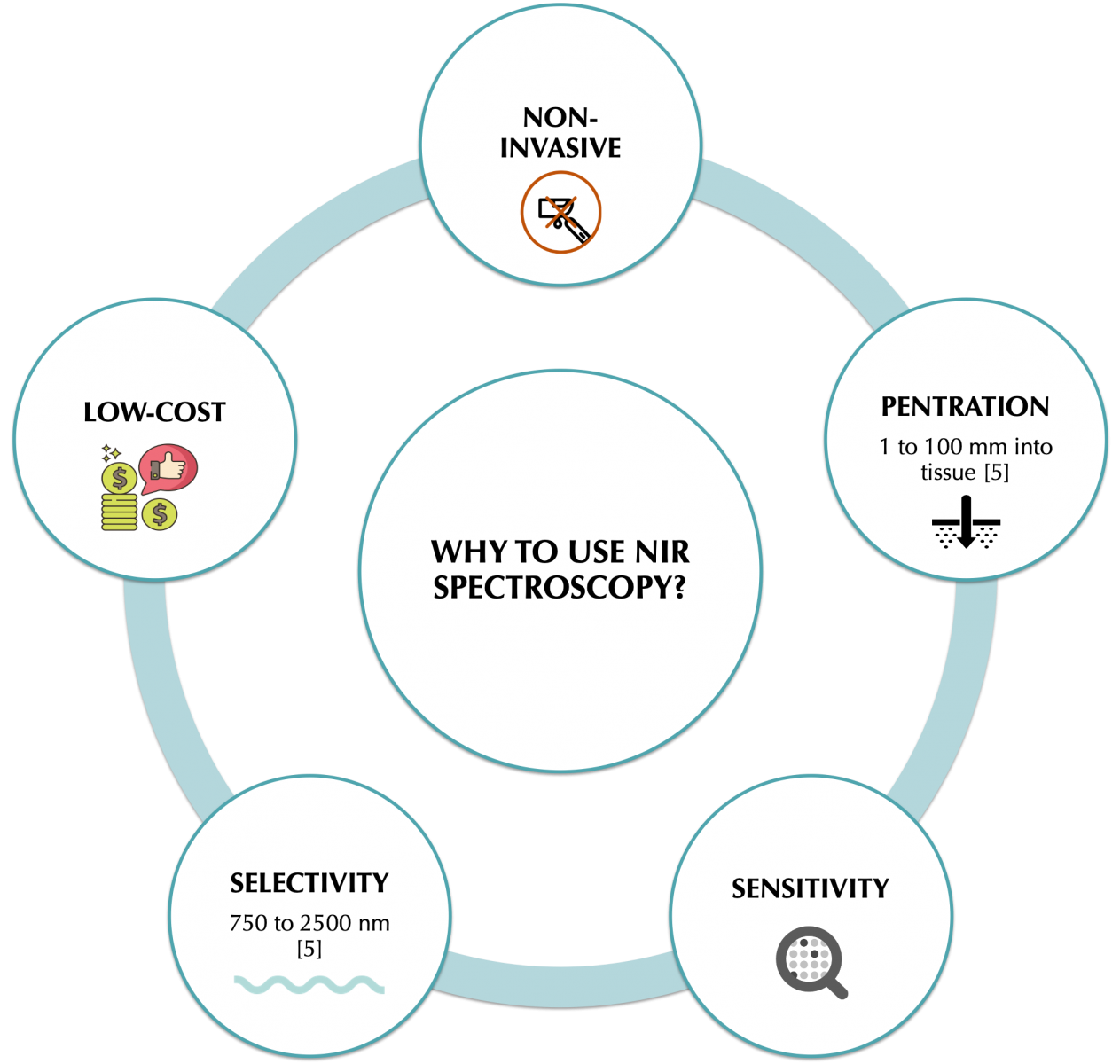
Several technologies are followed to develop glucose non-invasive sensors. The technologies are categorized into optical and transdermal sensors. Optical sensors possess a wide variety of approaches including Raman spectroscopy, fluorescence, photo-acoustic, thermal infrared, mid-infrared, and near-infrared [3].
NIR spectroscopy has been proved to be a good candidate method for monitoring not only glucose but many other physiological parameters [4].
 Measurement from the earlobe
Measurement from the earlobe
The area of the earlobe was found to possess the maximum glucose concentration due to the absence of bone tissues, its relatively small thickness, and its low amount of liquids [6].
The designing of the device is carried out into two stages through simulation and experiments.
- Building the system in a simulation environment:
The modified Beer-Lambert law will be applied to determine the concentration:
A = log (Io / I) = ε.l.C.DPF + G
d(A) = ε.l.d(C).DPF
- Building the system in the laboratory:
- The system will be designed using transmission and reflection setups to optimize the accuracy of the device, as represented in Figure 2.
- The proposed system will be tested on an earlobe tissue-mimicking phantom before examining it on a real subject.
- The results will be analyzed by comparing the obtained results with the measurements of a reliable calibrated device.
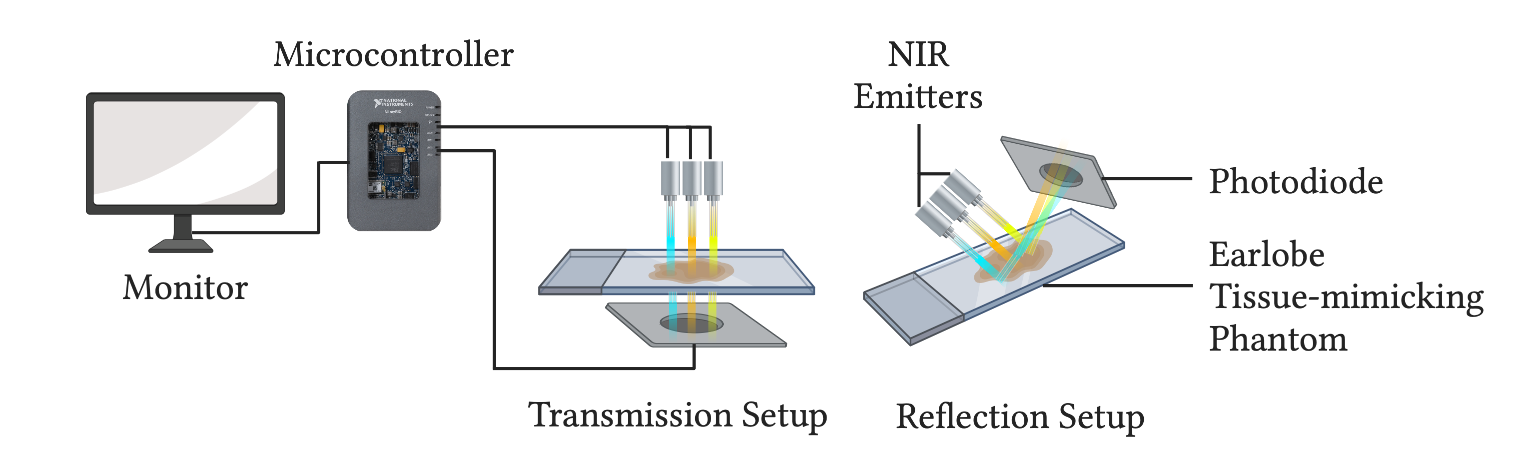
Figure 2: Schematic representation of the transmittance and reflectance modes of the system.
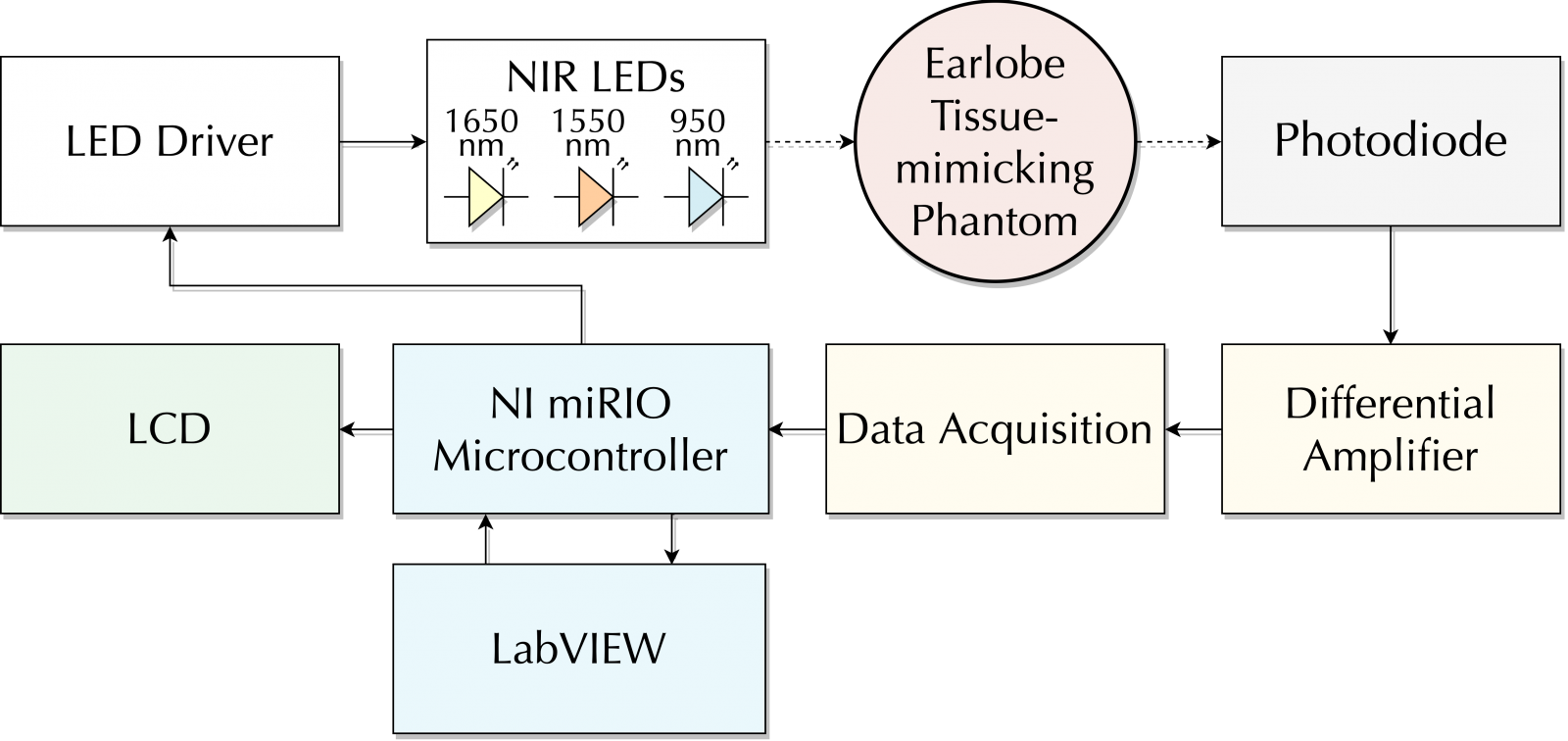
Figure 3: Block diagram of the proposed system.
To estimate the amount of power transmitted to the photodiode, the following preliminary results were calculated theoretically.
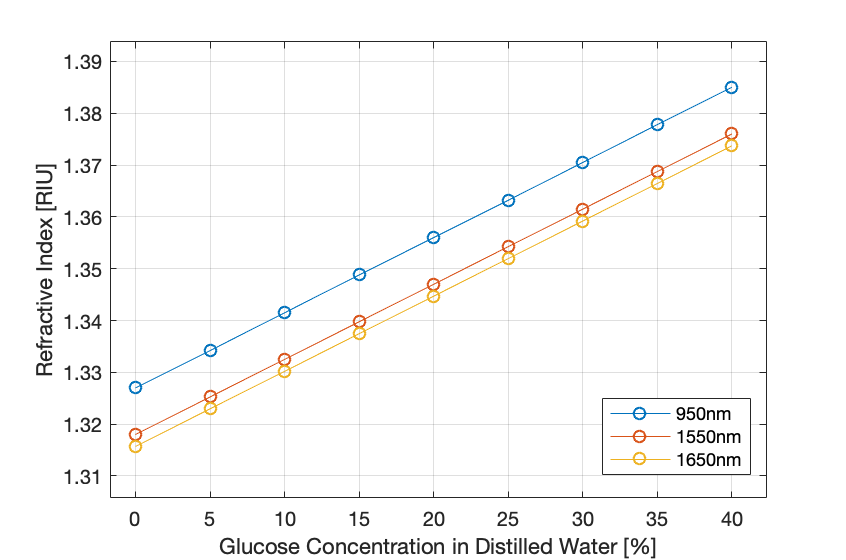
Figure 4: Refractive index of glucose solution with increasing the glucose concentration.
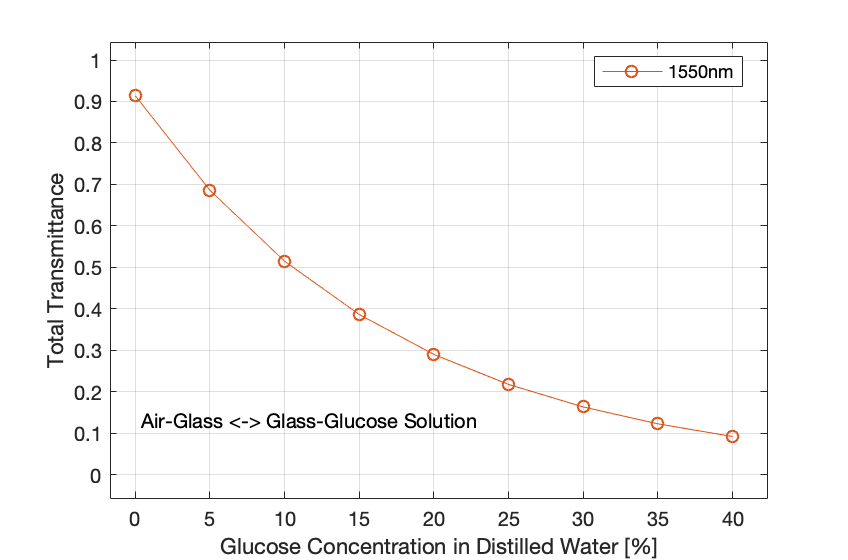
Figure 5: Total transmittance of light at a wavelength of 1550 nm to a glucose solution in a glass cuvette.
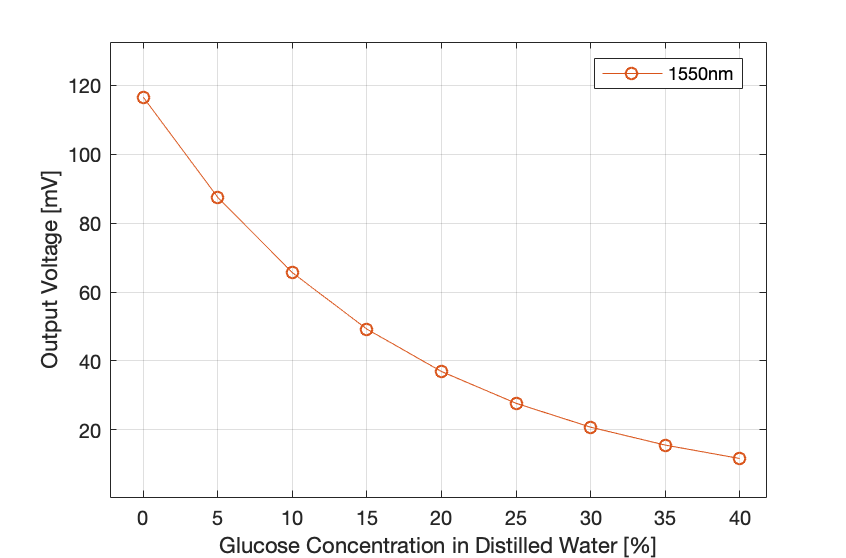
Figure 6: Output voltage at the detector versus glucose concentration at a wavelength of 1550 nm.
- The figures above show that the concentration of glucose in water is directly proportional to the refractive index and inversely proportional to the total transmittance of light at the detector along with the output voltage.
- The increase of the wavelength showed a decrease in the refraction of light through the sample.
- These preliminary results have been conducted without considering the scattering to prove the relationship between the refractive index and the glucose absorption and concentration.
The proposed non-invasive device is expected to surmount the limitations of low measuring accuracy level of the non-invasive glucometers due to its novelty in using multi-wavelengths of NIR light, and eventually replace the invasive traditional glucometer to revolutionize diabetic patients care.
Future Plans:
- Developing the earlobe tissue-mimicking phantom with the specific constitutes of the blood.
- Testing the prototype on a real human sample to check the result and prepare the final product.
- Making the device more compact in order to be wearable, as illustrated in Figure 7.
Figure 7: Expected prototype.
Special thanks and appreciation to the project supervisor, Dr. Ibraheem Al-Naib, for his advice, guidance, and encouragement throughout the development of the project.
[1] A. American Diabetes, "Diagnosis and classification of diabetes mellitus," Diabetes care, vol. 37 Suppl 1, no. 1, pp. S81-S90, 2014, doi: 10.2337/dc14-S081.
[2] "International Diabetes Federation - Home", Idf.org, 2017.
[3] N. Oliver, C. Toumazou, A. Cass and D. Johnston, "Glucose sensors: a review of current and emerging technology", Diabetic Medicine, vol. 26, no. 3, pp. 197-210, 2009.
[4] F. Fotouhi-Ghazvini, B. Javid and F. Zakeri, "Noninvasive optical diagnostic techniques for mobile blood glucose and bilirubin monitoring", Journal of Medical Signals & Sensors, vol. 8, no. 3, p. 125, 2018.
[5] J. Yadav, A. Rani, V. Singh, and B. M. Murari, "Prospects and limitations of non-invasive blood glucose monitoring using near-infrared spectroscopy," Biomedical Signal Processing and Control, vol. 18, pp. 214-227, 2015, doi: 10.1016/j.bspc.2015.01.005.
[6] W. Liu, A. Huang and P. Wan, "Overcoming Individual Discrepancies, a Learning Model for Non-Invasive Blood Glucose Measurement", Applied Sciences, vol. 9, no. 1, p. 192, 2019.
 Send Email
Send Email