


Theme
Nuclear Medicine
INSTITUTION
King Faisal Specialist Hospital and Research Center

Positron Emission Tomography (PET) is a present-day imaging modality used for clinical diagnostics and treatment managements. Also, PET is a functional imaging method that can detect biochemical and physiological alterations even before the arrival of anatomical changes. The interest in the development of labeling methods for organic and biological molecules with positron emitting radionuclides are becoming highly demandable as a wide range of uses of the PET imaging applications are increasing dramatically. An example, peptides labeled with positron-emitting radionuclides are of outstanding interest for the development of radiotracers that allow to image cellular functions in vivo.
Most biomolecules such as peptides, proteins and antibodies are not easy to be directly labelled due to their unstable structures and the difficulty in controlling critical reaction condition. Flourine-18 labeled compounds are more desirable because of its relatively longer half-life allows multi-step radiochemical manipulations as well as in vivo biological studies that can last for several hours.
N-Succinimidyl-4-[18F] fluorobenzoate (S[18F]B) is a well-known potential prosthetic agent that been used to conjugate with biomolecules to from corresponding [18F] fluorobenzoylated products for a variety of applications in nuclear medicine. S[18F]B can react with primary amines of biomolecules; it has been demonstrated to be a suitable and versatile F-prosthetic group to radiolabel peptides, proteins, and antibodies in terms of in vivo stability and radiolabeling yield. However, the drawback of flourine-18 labeling with biomolecules (antibodies, proteins and peptides) is that the radiosynthesis of the intermediate labelling agent (S[18F]B) involved a long synthesis time applying manual methodology. Thus, the reaction of S[18F]B with biologically relevant resin-bound peptides will be studied and optimized. For comparison, each peptide will be 18F-fluorobenzoylated in solution under different conditions and the product distribution will be analyzed proving the advantages of the solid-phase approach. The method’s feasibility for selective radiolabeling either at the N-terminus or at the lysine side chain shall be demonstrated.
Appling same steps that are well known to produce S[18F]B manually on the platform of the newly ALL IN ONE synthesizer was managed. Steps are summaries by drying, hydrolysis, activation and then purification. Modification is applied whenever necessary during our experiments. Below are two pictures of the ALL IN ONE synthesize and the platform steps that been built in order to produce S[18F]B.

ALL IN ONE synthesizer
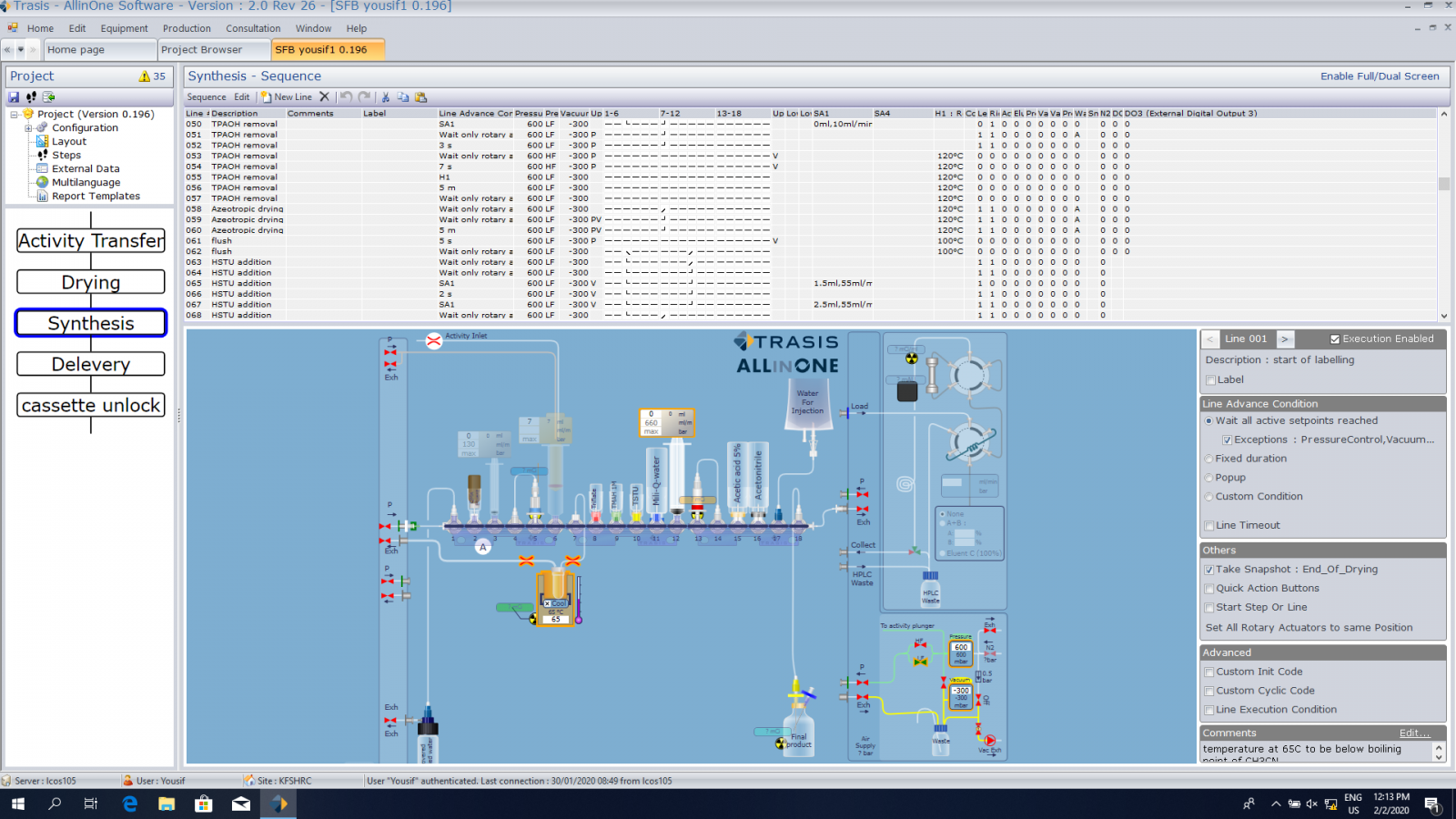
Building synthesis steps on the operational program
The S[18F]B is produced in one hour comparing to almost two hours using the manual methodology. Also through the presented methodology high to comparable radiochemical yields of the manual methodology (10-15%) are produced. Cleaner and higher radiochemical purity is also achieved.
The presented automation process is the first using the mentioned synthesizer and improving steps are ongoing to higher the radiochemical yields of the S[18F]B.
This work was supported by RAC # 2190 03..
Ganesan Vaidyanathan & Michael R Zalutsky, (Synthesis of N-succinimidyl 4-[18F] fluorobenzoate, an agent for labeling proteins and peptides with 18F), Published online 9 November 2006; doi:10.1038/nprot.2006.264.
G. Tang, Wenbin Zeng, Meixiang Yu, and G. Kabalka, (Facile synthesis of N-succinimidyl 4-[18F] fluorobenzoate ([18F]SFB) for protein labeling), Journal of Labelled Compounds and Radiopharmaceuticals 31 October 2007
Peter J. H. Scott and Xia Shao* (Fully automated, high yielding production of N-succinimidyl 4-[18F] fluorobenzoate ([18F]SFB), and its use in microwave-enhanced radiochemical coupling reactions), Journal of Labelled Compounds and Radiopharmaceuticals 12 April 2010.
Manuela Kuchar, Marc Pretze, Torsten Kniess (Site-selective radiolabeling of peptides by 18F-fluorobenzoylation with [18F]SFB in solution and on solid phase: a comparative study), Amino Acids (2012) 43:1431–1443.
Kang-Po Li , Ming-KuanHu, CliftonKwang-FuShen , Wei-YuLin , Shuang Hou , Li-BoZhao , Cheng-YiCheng, Daniel.H.Shen (Improved and optimized one-pot method for N-succinimidyl-4-[18F] fluorobenzoate([18F]SFB) synthesis using microwaves), Applied RadiationandIsotopes94(2014)113–117.
David Thonon, David Goblet, Eve Goukens, Geoffroy Kaisin, Jérôme Paris, Joël Aerts, Steve Lignon, Xavier Franci, Roland Hustinx and André Luxen , “Fully Automated Preparation and Conjugation of N-Succinimidyl 4-[18F]Fluorobenzoate ([18F]SFB) with RGD Peptide Using a GE FASTlab™ Synthesize”, Mol Imaging Biol (2011) 13:1088-1095.
 Send Email
Send Email